
PALM BEACH, FL – Yesterday, September 2, 2021, Pharmacy Times, a monthly journal for pharmacists, published the results of a study which analyzed incidents of Bell’s palsy related to two approved vaccines in Hong Kong, the “Pfizer-BioNTech” (BNT162b2) vaccine and the “CoronaVac” (Sinovac Biotech) vaccine, both of which are designed for the prevention of COVID-19 in humans. The study suggests a “small” yet “increased risk” of Bell’s palsy associated with vaccination.
The study was necessary as there appears to be rising cases of Bell’s palsy in India according to Dr Vinith Karanth, Consulting Physiotherapist at Zen Multispecialty Hospital.
“Since last year, there is a drastic increase in the number of Bell’s palsy cases coming to the physiotherapy department, particularly patients with a history of COVID-19 infection. In the last few months, around 3-4 post-Covid patients, both young and old, are appearing with this problem on a daily basis. Before Covid, we used to see such cases appearing very rarely, 1-2 cases in a month.
Dr Vinith Karanth, Consulting Physiotherapist at Zen Multispecialty Hospital.
Additionally, a May 13, 2021, publication in the National Library of Medicine’s National Institutes of Health (NIH) stated both the Moderna and Pfizer Vaccine trials reported Bell’s Palsy as adverse events.
The study was conducted by researchers from The University of Hong Kong and found that for every 100,000 people vaccinated, approximately 5 (4.8) people may develop the condition.
“Our study suggests a small increased risk of Bell’s palsy associated with CoronaVac vaccination,” said Professor Ian C.K. WONG, Co-Director of the Centre for Safe Medication Practice and Research, Department of Pharmacology & Pharmacy, and holder of the Lo Shiu Kwan Kan Po Ling Professorship in Pharmacy at HKU. “Nevertheless, Bell’s palsy remains a rare, mostly temporary, adverse event. All evidence to date, from multiple studies, shows that the beneficial and protective effects of the inactivated COVID-19 vaccine far outweigh any risks. Ongoing surveillance, through pharmacovigilance studies such as ours are important to calculate with increasing levels of confidence the risks of rare adverse events.”
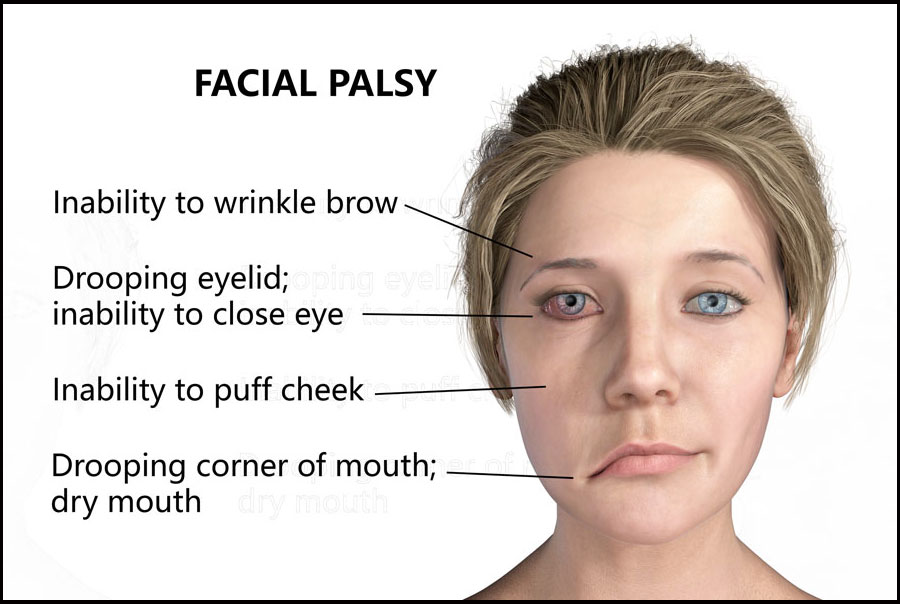
Bell’s palsy is an unexplained episode of facial muscle weakness or paralysis which usually causes a drooping of one side of the face. It begins suddenly and worsens over 48 hours. Most patients show some recovery without intervention in 2–3 weeks and most cases resolve within 3–4 months.
However, COVID-19 vaccines specifically may not be the only vaccines that cause such an adverse effect. According to a July 21 2020 publication in the NIH, a four year study conducted from January 2015 to October 2019 found that influenza type vaccinations have shown an increased number of facial paralysis when compared with other vaccines.
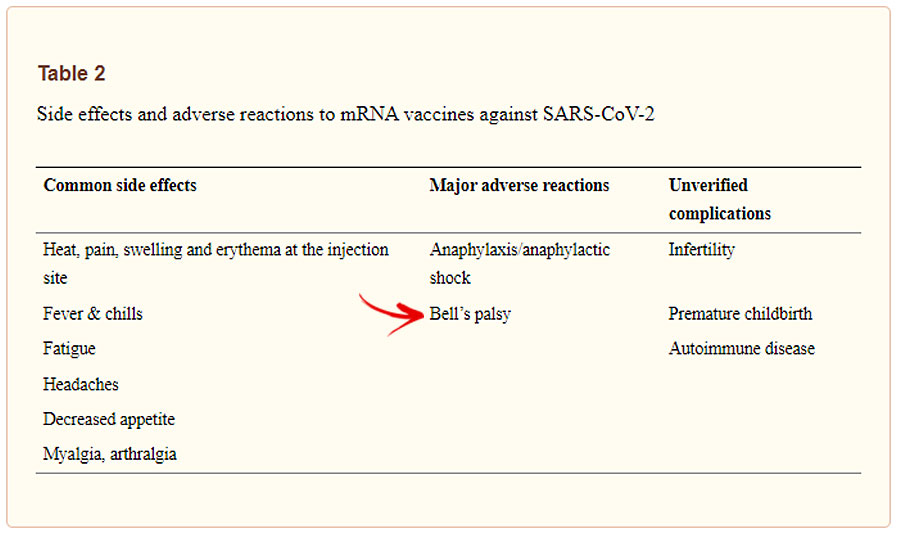
While the condition was reported as extremely rare considering the numbers of vaccines administered compared with the numbers of people who develop the condition [CoronaVac (3.61 cases per 100,000 doses) Pfizer-BioNTech (2.04 cases per 100,000)], a May 2021 NIH review of the safety of COVID-19 mRNA vaccines listed Bell’s palsy as major side effect.
Following what it calls the first large-scale population-based study, weekly peer-reviewed medical journal The Lancet concluded that the association between the SARS-CoV-2 vaccines and Bell’s palsy confirms that the beneficial and protective effects of the vaccines far outweigh the risk of this rare adverse event. The Lancet is of considered “the gold standard” in medical reporting and often cited as one of the oldest and most respected medical journals in the world. Oddly enough, one of the largest retractions in history was a publication and then retraction of information on the controversial drug, hydroxychloroquine.
Nonetheless, last month, governmental department Health Canada, which is responsible for national health policy in Canada announced that it decided to update the labelling for the Pfizer-BioNTech COVID-19 vaccine to acknowledge the very rare reports of Bell’s palsy following vaccination. Health Canada continues to assure Canadians the vaccines are safe and effective in protecting against COVID-19, and that the benefits of being vaccinated far outweigh the risks.
NIH references and further reading:
- Bell’s Palsy after second dose of Pfizer COVID-19 vaccination in a patient with history of recurrent Bell’s palsy
- Interpretation of vaccine associated neurological adverse events: a methodological and historical review
- Sequential contralateral facial nerve palsies following COVID-19 vaccination first and second doses
- SARS‐CoV‐2 vaccines are not free of neurological side effects
- Covid-19-vaccine-Pfizer-BioNTech/prednisone, Hypertension, injection-site soreness and recurrence of Bell’s palsy: case report



Comments are closed.